We help companies
bring innovative products to market in controlled environments
At Malsen, we have extensive experience of all stages of the product and company life-cycle; from the initial product conception, industrialisation, through to local and global market launch with our support all of the way. We enjoy complex technical and political challenges in controlled environments, where the best practices need to be deployed company wide.
Work with ambitious individuals, collaborate on meaningful projects, and find exceptional solutions.

Our clients range from large multinationals, through to small one person startups. All of which need to find efficient ways to comply to the latest regulations for medical and pharmaceutical products.
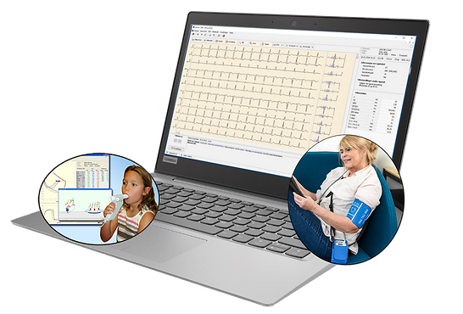
Diagnostica
A medical IT company that markets the product Spirare used by health care professionals to visualize and manage data gathered from several modalities.
Malsen Medical created the technical file for compliance to MDD and associated QMS. Full compliance to IEC 62304 in context of MDR with a certified ISO 13485 QMS will help ensure continued business stability and growth.
Pharmasens
Pharmasens, a Swiss based company developing a groundbreaking insulin delivery platform.
Malsen Medical is helping to drive the project is several areas including risk management, specification and requirement management, compliance planning, FDA submission preparation, labelling, verification testing, QMS and other areas. The device has now been submitted and passed FDA validation.

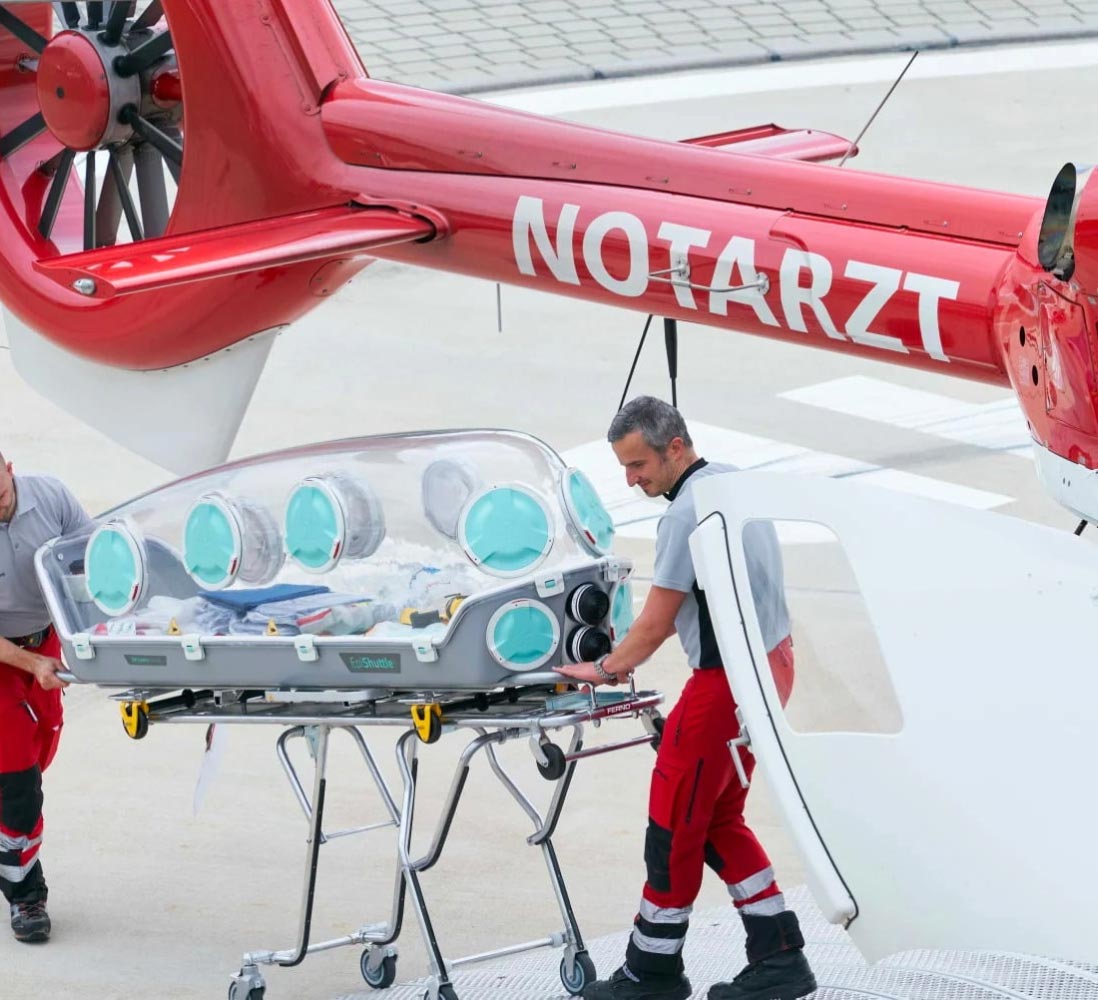
EPIGuard
EpiGuard. EpiShuttle is a medical isolation and transportation system designed for optimal safety and numerous treatment options during patient loading and transport. The unit is a single-patient isolator made of solvent-resistant materials. EpiShuttle can be carried and is compatible with leading ambulance-stretcher undercarriage EMS systems.
Malsen Medical have been Involved with EpiGuard for several years to set up their production, regulatory strategy, QMS and technical documentation.
HAT
The HAT is based on an idea by the Norwegian Air Ambulance Foundation (SNLA). Their paramedics and rescuers were lacking a device that would immediately start and activate heat treatment, whilst leaving them room for treating the patient. The development is currently coordinated with SNLA and Minitech AS, a Norwegian leader in medical device heating systems.
We are very proud to have supported the Norwegian Air Ambulance and Minitech AS with the engineering and regulatory submittal for this project through all stages of development and MDR submission in just 2 years.


Pharmaceutical Serialization
The falsification directive (FMD) was issued in 2011 and came into force on 9 Feb 2019 to ensure that all prescription medicines sold in Europe are verified to be authentic prior to being sold. The system is a pan-European IT system that affects all involved in the pharmaceutical industry.
Malsen Medical has been working on this project from around 2015 in various roles. We worked both in testing the system globally prior to launch and building up the QMS systems locally in several countries and fostering pan-European collaboration. We continue to support and develop the system further in Europe and now also in Africa.
